Great management can take care of well-being in an expert organization even under exceptional circumstances.
Read the article →Responsibility has become a real Big Question in industry in the past few years. As we become more environmentally aware, companies have a rather conflicting role in it: They can innovate solutions that contribute to...
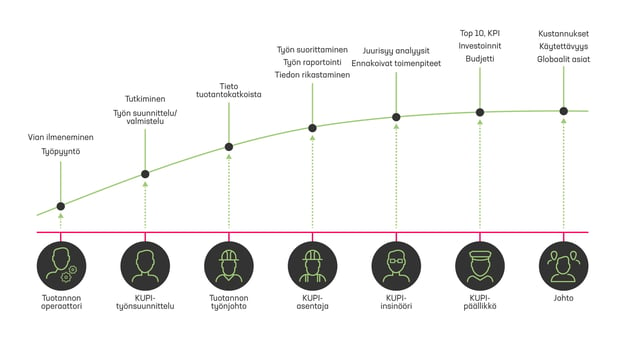
Read the article →Maintenance data is enriched on its way from the technician’s task schedule to the desk of the maintenance manager or even the factory manager.
Read the article →The total number of personnel at Pinja’s Tampere offices has doubled in two years – nearly 100 employees already! Among other things, the office has wall art devised by the staff and realized by professionals.
Read the article →Decisions made in a change situation influence manufacturing designers, the ground-level staff, production management, material procurement, sales, financial department and ultimately also the company management.
Read the article →From the cultural point of view, it is important to consider how to introduce new tools as smoothly as possible while causing as little disturbance as possible to users’ daily work.
Read the article →Improving productivity requires an in-depth understanding and insight into policies and their impact on efficiency.
Read the article →The EU’s Renewable Energy Directive, the so-called RED III Directive, steers the national legislation of the member states in an increasingly environmentally conscious direction. In this blog, we discuss the background...
Read the article →Microsoft 365 is a modern cloud service toolpack that adapts to various needs and different user profiles.
Read the article →Acquiring a production planning system is a significant future investment for a business in the manufacturing industry. The system supplier will provide support during all stages of the procurement process, but the...
Read the article →